The Proof that the U.S. Government
Supported a Vexatious Litigant
in Defrauding the Defendant and the Court

The ink had barely
dried on the author's May 26, 2004 coerced plea agreement
when May 29, three days later, Sue Gilliatt signed a
4-page sworn affidavit identifying the
co-defendent, Dan Raber, as the sole source of all her
medical troubles. It was then entered into United States
District Court, Southern District of Indiana, on June 3, 2004.

This document can be viewed
at right by clicking any one of the four pages
to see their enlargement. The reader can see that
no where in this document does my name,
or that of Alpha Omega Labs, appear.

It was not a problem
for the Federal Court that Ms. Gilliatt's affidavit is
inconsistent with her testimony that the Alpha Omega Labs
product was the primary cause of her injury . . . or
her
deposition testimony
the following July --
made prior to sentencing.
As soon as my pleading was complete,
Gilliatt and her lawyers were assured of collecting
what would end up amounting to
$800,000 off
Alpha Omega Labs' product liability insurance policy.
And so . . . with their first victory in hand, it appeared
to them that the next logical thing to do was not argue
with success: go after another defendant and claim that
HIS product was the sole source of her medical condition.
(The simply fact is that if you
have a medical doctor surgically remove your nose, then
THAT is the cause of the removed nose -- which
is what actually occurred.)

If
an objective observer compares the Affidavit at right
with Sue Gilliatt's
deposition of July 22nd,
one will find mind-numbing inconsistencies. For example,
let's take her experience with Cansema and a bloodroot paste
from another herbal company (Appalachian) as it appears
in her deposition,
from p. 48-92. She states that she applied Cansema on
September 21, 2001, and with repeated applications by
September 22, 2001 had extensive damage to her nose.
But in both the Affidavit, at right, and in her Deposition,
she testifies that on
September 24, 2001, she applied Raber's Bloodroot Salve,
a product she acknowledges as being similar in nature
in her deposition. If you take a product that you claim
to have done very extensive damage to your face, why would you
turn around and use a like product just two days later ---
especially if you work as a professional nurse and are
surrounding by medical practitioners?

Amazingly,
she DOES admit on p. 84 of her deposition that
she believes that a "combination of both of the products"
removed her cancer -- that is, made her cancer-free.
Yet, amazingly, she makes the claim that the
Cansema
testimonials from
OTHER people that we collected
from delighted end users were "fictionalized or falsified
in order to support (their) claim." (p. 86, L. 20).
She admits she has no evidence for this claim, other than her personal
opinion (p. 86, Ll. 21-24). Yet it is on this opinion,
among other factors, that the FDA claims to have acted
against the author personally.

(Truth is, not a single
testimonial was ever "doctored" in the history of
Alpha Omega Labs.

I should know.

I transcribed
and posted each and every one of them as we received them.)

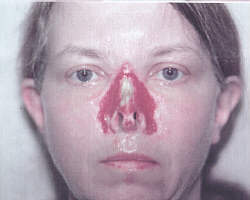
There is no herbal
salve, sold anywhere in the world --- not from the
author, not from Dan Raber, not from any vendor of
escharotics with which the author is or has been
familiar, that will do to
Sue Gilliatt's nose what can clearly be seen in
the photograph at the top of this page.
The clear, perfect, symmetrical cut lines are clear
evidence that the nose was "doctored."
The passage of time and the examination of the evidence will,
in time, show the world what I already know: that
Gilliatt lied under oath about the timing of her
photograph. It was taken
AFTER and not
BEFORE surgical intervention.

With no medical
insurance policy to go after,
Dan Raber -- who
was civilly accused of the very same things that the author was,
settled the matter for $10,000 to get rid of the legal
nuisance. The FDA came by at a later date to pick his remaining
"unauthorized" inventory of product.

Dan Raber was never
the subject of a Grand Jury hearing. Nor was he ever
charged, arrested, or imprisoned. His home and business
were not raided and sacked. His wife, child, and employees
were not threatened.

Had Kevin Trudeau
been paying FDA Agent, John Armand, to follow through on a "hit,"
it would have been an entire different matter
for Dan Raber and his company.

The complicity of
the U.S. Attorney's Office is clear and unmistakeable.
Long after the Defendant's counsel made clear the
discrepancy in U.S. Federal Court in Lafayette, the
U.S. Attorney's office continued to support the notion
that
Cansema® unceremoniously ripped Sue Gilliatt's
nose off, creating perfect surgical markings in the process.
(The claim is not made on the Dept. of Justice
web site, but
in statements direct from the
U.S. Attorney).
A good example of this brazen tendency
to keep the lie that initiates a prosecution going
is that made by U.S. Attorney Larry Regan (who has
since retired and now works for the City Attorney's
office in Lake Charles). He states in
one interview that
"two women, one in Indiana and another in Texas, suffered
injuries after using . . . Cansema." This claim
was untruthful when U.S. Attorney Larry Regan made it,
and he knew full well that it was a lie when he
made it to the media. Sharon Lee (the Texas case)
never even used Cansema, and Sue Gilliatt's
case was immaterial: the basis for the plea agreement
involved H3O and Cansema Tonic III,
products that neither
women had ever used. No mention of the use of
Dan Raber's product
is made. No mention is made that both products carried
the DSHEA disclaimer, "To U.S. Users: this product is not
intended to diagnose, treat, prevent, or cure any disease."
No mention is made that Gilliatt used the product
exactly as we have instructed customers
not to.

U.S.
Justice apologists will say that
Regan's statement that
Cansema was "sulfuric acid"
was just a slip . . . that he must be referring to
H3O. But even
THAT is an untruthful statement.
Just as is the claim that it was not a thoroughly tested
product -- which it decidedly was (by the California
manufacturer that made the product, supplied us with the
claims, and provided the majority of our testimonials
on that product.)

It is this consistency of
untruthful behavior that one finds in everything the
Department of Justice does -- or as Gore Vidal notes,
"When a U.S. prosecutor enters a federal court,
perjury is his native tongue."

In or
OUT
of the courtroom, fabrication on-the-fly to suit the desired
appearances of the moment is the order of the day.

Such is the sorry
state of the criminal justice system in the U.S. today ---
as expounded and documented extensively in the work of
Rodney Stich.
The Affidavit
(click to enlarge)
| 



